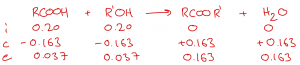You will usually be introduced to titration and back titration through the context of inorganic chemistry e.g., acid-base, redox and complexometric titrations.
However, titrations can also be useful in organic chemistry! In the example below, a titration is used to determine the amount of ester produced in an esterification reaction:
| Question 1: 0.20 mol of a carboxylic acid (RCOOH) and 0.20 mol of an alcohol (R’OH) are heated under reflux with 0.030 mol H2SO4. The mixture is then titrated with 48.5 cm3 of 2.0 mol dm-3 NaOH. Determine the amount of ester formed. Answer The first step is to work out how many moles of NaOH are required in the titration: n NaOH = 48.5 / 1000 x 2.0 = 0.097 mol The titration actually involves two seperate reactions, with the NaOH reacting with both the H2SO4 catalyst and any unreacted carboxylic acid: Reaction 1: H2SO4 + 2 NaOH –> Na2SO4 Reaction 2: RCOOH + NaOH –> RCOONa + H2O Since sulfuric acid is a catalyst, there will still be 0.030 mol H2SO4 in the final mixture. This means that 0.060 mol NaOH is required to neutralise the H2SO4 catalyst, leaving the remaining NaOH (i.e., 0.097 – 0.060 = 0.037 mol) to react with the carboxylic acid in a 1:1 ratio. This means that the amount of carboxylic acid remaining at equilibrium was 0.037 mol. Using all this information, we can complete an ICE table for the esterification reaction: As shown by the ICE table, if the amount of the carboxylic acid (RCOOH) decreases by 0.163 mol, the amount of ester (RCOOR’) must increase by 0.163 mol. The final amount of ester in the product mixture is therefore 0.163 mol. |