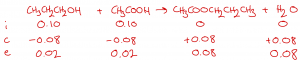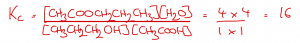If you are given the initial amounts of substances, the balanced symbol equation and the final amount of one substance, you can work out the final molar amounts of ALL substances using an ICE table.
| Question: 6.00 g of ethanoic acid (CH3COOH) and 6.00 g of propan-1-ol (CH3CH2CH2OH) are heated under reflux with H2SO4. The amount of propyl ethanoate (CH3COOCH2CH2CH3) formed is 8.16 g. The total volume of the mixture is 20 cm3. Determine the value of Kc under these conditions. Answer The balanced symbol equation for this esterification reaction is: CH3CH2CH2OH + CH3COOH –> CH3COOCH2CH2CH3 + H2O We can calculate the initial moles of ethanoic acid and propan-1-ol by dividing their mass by their Mr. The Mr of both is 60. initial CH3COOH = 6.00 / 60 = 0.10 mol initial CH3CH2CH2OH = 6.00 / 60 = 0.10 mol We can also assume that the initial moles of propyl ethanoate and water (i.e., the products) are both zero. initial CH3COOCH2CH2CH3 = 0 initial H2O = 0 We can also calculate the end moles of propyl ethanoate by dividing by its Mr (102). end CH3COOCH2CH2CH3 = 8.16 / 102 = 0.08 mol Using an ICE table, we see that the change moles of propyl ethanoate was +0.08 mol. Also, using the molar ratios in the balanced symbol equation, we can complete the change moles for the other substances involved, and hence, calculate the end moles for all substances.
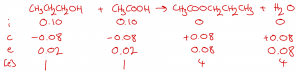
Kc is defined in terms of concentrations (rather than amounts in moles) so next we divide each of the molar amounts by the total volume (20 cm3 = 0.020 dm3) to determine the equilibrium concentration [e] of each substance:
Finally, we can substitute the calculated equilibrium concentrations into the Kc equation for this reaction:
In this example, Kc is unitless because there are 2 molecules on each side of the equilibrium. This means that the units of concentration will completely cancel. Furthermore, because the units of concentration cancel, the units of volume will also cancel. This means that, for this reaction, it is not necessary to known the total volume. |