A very short post on a very useful formula:
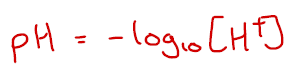
The concentration of H+ ions in solutions vary by many orders of magnitude but the pH equation allows us to express this very quickly using convenient numbers (typically between -1 and 15 ish). This equation means that if the [H+] increases by a factor of 10, the pH decreases by exactly 1.
Let’s say we want to find the pH of solution with [H+] = 0.1 mol dm-3. We would enter -log(0.1) or -log10(0.1) into our calculator and get an answer of 0.30. You should always give pHs to two decimal places because this is what a digital pH probe will always give it as.
A related question is then how do we work out [H+] for a given pH? If you are doing A Level Physics or A Level Maths, you’ll probably know already, if not here’s the formula:
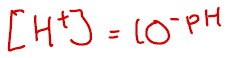
To enter this into your calculator, you’ll need to use the x[] or 10[] buttons, or something very similar. A solution with a pH 3.45, has [H+] = 10-3.45 = 3.55 x 10-4 mol dm-3. Hopefully you got this!
NOTE – The acids and base topic will NOT use the ln and e[] buttons so don’t get mixed up. You will use these buttons on the Kinetics topic, and also much more frequently in A Level Physics or A Level Maths.