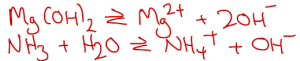Bases are proton acceptors by definition. They produce OH– ions in aqueous solution, either by accepting a H+ from water, or by the base itself containing OH– ions.
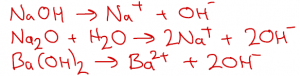
All the examples above are strong bases because the reactions go to completion. This means we can work [OH–] simply by knowing the initial amount of base and the volume of the solution. From [OH–], we can then use the Kw equation to find [H+] and then the pH.
| Question: 0.40 g of NaOH is dissolved into 2000 cm3 of distilled H2O. Calculate the pH of the solution formed at 25 oC Kw = 1.00 x 10-14 mol2 dm-6. Answer: The first task is to work out the moles of NaOH, using its mass and its Mr (40) n NaOH = 0.40 / 40 = 0.01 mol The dissolving goes to completion so there is a 1:1 relationship between initial NaOH and final OH–, so we can work out the final moles of OH- and then calculate its concentration using the volume (2000 cm3 = 2 dm3) n OH– = 0.01 mol [OH-] = 0.01 / 2 = 0.005 mol dm-3 The next step is to write down the Kw equation and re-arrange to make [H+] the subject: Kw = [H+][OH–] [H+] = Kw / [OH–] = (1.00 x 10-14)/0.005 = 2 x 10-12 mol dm-3 Finally, we use the pH equation to find that the pH = -log(2 x 10-12) = 11.70. |
In contrast to the examples above, weak bases produce OH– ions in aqueous solution through reversible reactions that do not go to completion. To determine the pH of these solutions we would also need to know something about the relevant equilibrium constants: