The strong acid assumption is that the acid (HA) in solution dissociates completely into ions (i.e., H+ and A–).
This assumption is useful because we can work out the end [H+] and pH simply from the concentration of acid that we start with and we don’t have to worry about any equilibrium constants. An ICE table can be useful to see what’s going on (but probably unnecessary):
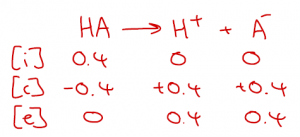
So, if I have a bottle of HCl solution with 0.4 mol dm-3 HCl written on it, it is actually a solution containg 0.4 mol dm-3 H+ and 0.4 mol dm-3 Cl–. There will be very few molecules of undissociated HCl left. The pH of this solution is -log(0.4) = 0.40
We use the same approach for strong diprotic acids such as H2SO4. Here, an ICE table is a good reminder of the fact that complete dissociation of H2SO4 produces 2 H+ ions.
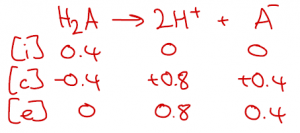
So, if I have a bottle of H2SO4 solution with 0.4 mol dm-3 H2SO4 written on it, applying the strong acid approximation suggests that I actually have a solution containg 0.8 mol dm-3 H+ and 0.4 mol dm-3 SO42-. The pH of this solution would be -log(0.8) = 0.10
(The strong acid assumption actually gets a little shaky here. In reality, the first dissociation of H2SO4 is complete but the second dissociation of H2SO4 is much more partial. A solution of H2SO4 therefore actually contains mostly H+ and HSO3–, so there is a lower [H+] and higher pH than expected)