One of the first facts that Year 7 students learn in Chemistry is that water has a pH of 7. However, this isn’t some fundamental law of the universe, but simply reflects the value of an equilibrium constant at 25 oC.
Water contains H+ and OH– ions, formed by partial dissociation of water molecules. This is a reversible reaction:

We can write a Kc expression for this. Since the dissociation of water is so small and [H2O] is effectively constant, we can multiply both sides by [H2O] to form a new constant, Kw.
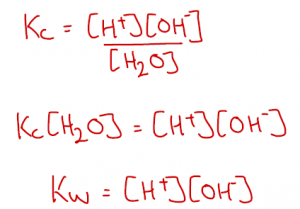
The equilibrium is endothermic so as temperature rises, the equilibrium shifts right to increase [H+] and [OH–], so Kw increases with increasing temperature.
Everything so far is completely general and applies to any solutions containing water (i.e., acids, alkalis, neutral).
However, in a neutral solution such as pure water we can also say that [H+] = [OH–] which allows further simplifiying.
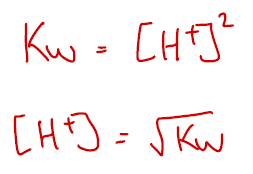
The value of Kw at 25 oC is 1.0 x 10-14 mol2 dm-6. This means that [H+] = 1.0 x 10-7 mol dm-3. Using the pH equation below, this gives a pH of pure water at 25 oC as being 7.00.
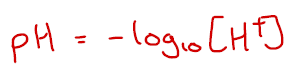
At higher temperatures, Kw increases which means [H+] increases. This means that pH decreases, even if the solution is still neutral (defined as when [H+]=[OH–])
As a specific example, Kw = 5.5 x 10-14 mol2 dm-6 at 50 oC, so [H+] = 2.35 x 10-7 mol dm-3, and pH 6.63. The solution is still, of course, neutral.